Table of Contents
- Executive Summary and Key Insights for 2025
- Technological Overview: How Backward Wave Doppler Imaging Systems Work
- Current Market Landscape: Key Players and Regional Hotspots
- Emerging Applications: Clinical, Industrial, and Research Uses
- 2025-2030 Market Forecast: Revenue, Volume, and Growth Drivers
- Innovation Pipeline: Patents, R&D, and Next-Gen System Features
- Competitive Analysis: Strategies from Leading Manufacturers (e.g., siemens-healthineers.com, gehealthcare.com, philips.com)
- Regulatory and Standards Outlook: Approvals, Safety, and Compliance (e.g., fda.gov, ieee.org)
- Investment & Partnership Trends: Funding, M&A, and Collaborations
- Future Outlook: Challenges, Opportunities, and Disruptive Potential through 2030
- Sources & References
https://youtube.com/watch?v=LiFiNCFEzhQ
Executive Summary and Key Insights for 2025
The field of Backward Wave Doppler Imaging Systems is poised for notable advancements and market activity in 2025, fueled by ongoing innovation in ultrasound technology, signal processing, and clinical demand for enhanced diagnostic modalities. These systems leverage the phenomenon of backward wave propagation to achieve higher resolution and more precise velocity measurements in cardiovascular and vascular imaging, addressing key limitations of conventional Doppler approaches.
- In 2025, leading ultrasound system manufacturers are intensifying efforts to integrate backward wave capability into next-generation diagnostic platforms. GE HealthCare and Philips are notable for their investments in advanced Doppler and imaging technologies that enable more sensitive detection of flow disturbances, supporting earlier intervention in cardiac and peripheral vascular conditions.
- Backward wave Doppler techniques are increasingly being explored for their potential to quantify arterial stiffness, a key biomarker in hypertension and atherosclerosis. Research collaborations with clinical partners—such as those announced by Siemens Healthineers—signal that 2025 will see pilot implementations in major hospitals and research centers, laying the groundwork for broader clinical adoption.
- Component suppliers are responding to this trend with dedicated transducer and signal processing chipsets. Teledyne and Analog Devices are among those introducing hardware optimized for high-frequency, high-fidelity Doppler measurements, addressing the technical demands of backward wave imaging.
- Regulatory activity is expected to accelerate in 2025. The U.S. Food and Drug Administration (FDA) and European regulatory bodies have signaled support for streamlined review of novel Doppler technologies, with several companies announcing pre-market submissions and clinical validation studies.
- Looking forward to the next few years, the outlook is characterized by a convergence of hardware innovation, improved software algorithms, and growing clinical validation. The emergence of AI-assisted Doppler analysis, as seen in product pipelines from Canon Medical Systems, is expected to further enhance accuracy and usability, supporting broader deployment in both high-end and point-of-care ultrasound systems.
In summary, 2025 marks a pivotal year for Backward Wave Doppler Imaging Systems, with momentum building around clinical integration, technical innovation, and regulatory progress. This positions the sector for robust growth and expanded impact on patient care through the rest of the decade.
Technological Overview: How Backward Wave Doppler Imaging Systems Work
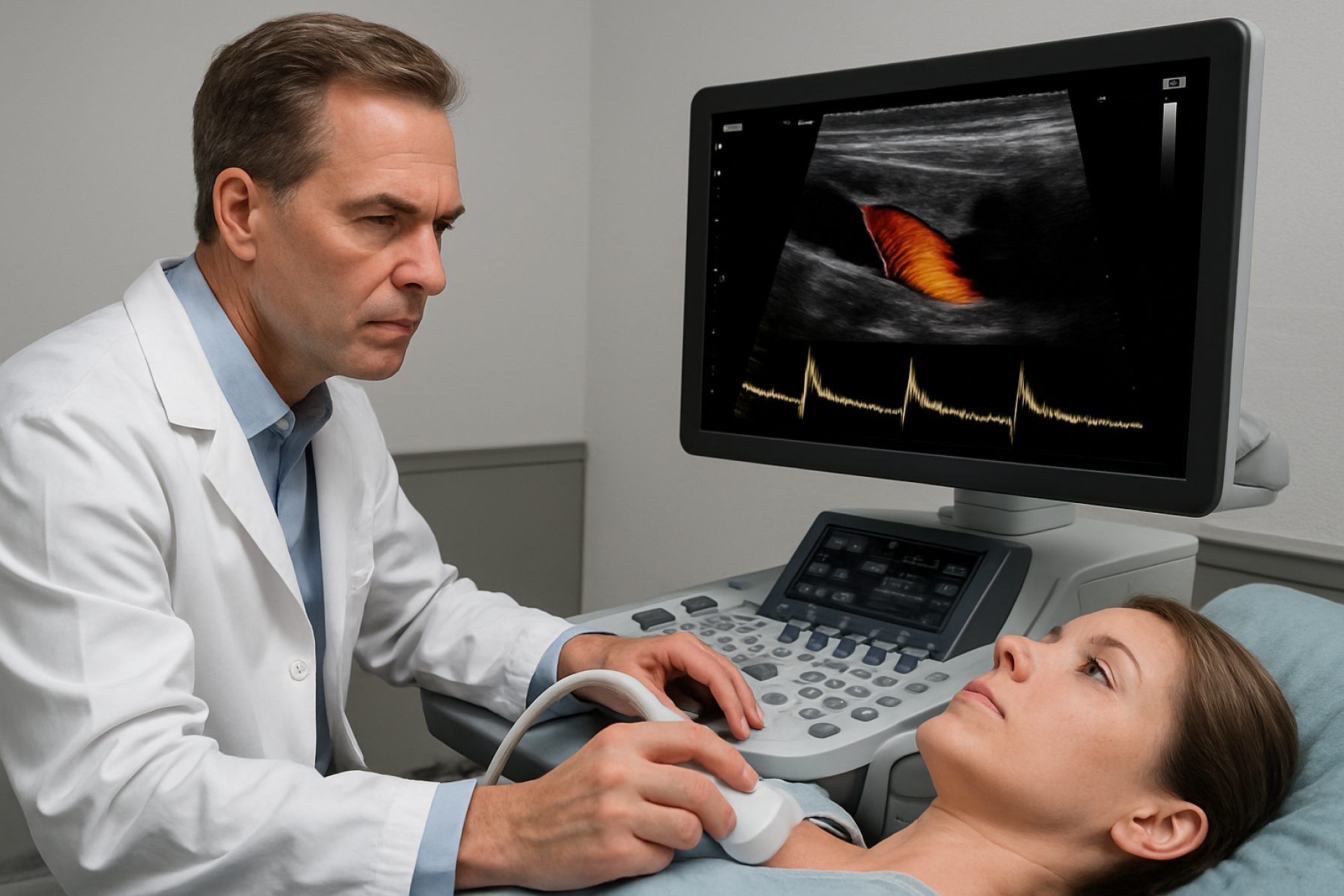
Backward Wave Doppler Imaging Systems represent an advanced evolution in Doppler ultrasound technology, leveraging the phenomenon of backward (or reverse) wave propagation to enhance sensitivity and resolution in flow measurement and imaging. Unlike traditional Doppler systems that primarily analyze forward-scattered waves, these systems utilize the unique properties of backward-propagating acoustic waves, often generated via nonlinear interactions in specially engineered transducer arrays or metamaterials.
At the core of these systems are transducer technologies capable of both generating and detecting backward-propagating waves. This typically involves the use of piezoelectric materials with precise geometric configurations, or the implementation of acoustic metamaterials designed to support phase-conjugation or negative refractive index behaviors. Companies such as Olympus Corporation and Siemens Healthineers have developed highly sensitive transducer arrays that are integral to the field, though the explicit integration of backward wave modalities remains at the forefront of ongoing research and prototype development, with first clinical or preclinical systems expected to emerge within the next few years.
Functionally, backward wave Doppler imaging operates by emitting ultrasound pulses into the tissue and then analyzing the echo signals that return not only from conventional forward scattering, but also from backward-propagating modes. These backward modes provide additional spatial and velocity information, particularly in complex flow environments such as turbulent or multidirectional blood flow in cardiovascular diagnostics. The signal processing chains are enhanced using advanced algorithms—often employing machine learning and real-time spectral decomposition—to differentiate and interpret signals arising from backward propagation. Leading imaging platform providers like GE HealthCare and Philips are actively incorporating AI-driven processing modules into their next-generation ultrasound systems to facilitate such advanced imaging modalities.
Current research and pilot deployments in 2025 are focusing on the validation of these approaches in both laboratory and clinical settings, with early data suggesting significant improvements in the detection of microvascular flows and complex hemodynamics. As component miniaturization and real-time processing capabilities continue to advance, the outlook for backward wave Doppler imaging is highly promising, with increasing interest from manufacturers and healthcare providers aiming for commercialization and routine clinical use within the next few years.
Current Market Landscape: Key Players and Regional Hotspots
The market for Backward Wave Doppler Imaging Systems in 2025 is characterized by heightened innovation, increasing clinical adoption, and an expanding competitive landscape. Key players in this sector are predominantly established ultrasound and medical imaging companies leveraging advancements in signal processing and transducer design to enhance Doppler sensitivity and accuracy.
- Key Players: Notable manufacturers include GE HealthCare, which continues to integrate backward wave Doppler capabilities into its premium ultrasound platforms, and Siemens Healthineers, whose recent product releases highlight improved backward flow detection relevant to cardiovascular diagnostics. Philips Healthcare also invests in backward wave Doppler technology as part of its high-end EPIQ and Affiniti ultrasound series. Japanese firms such as Hitachi and Canon Medical Systems Corporation are actively developing proprietary algorithms to refine the quantification of backward flow in vascular imaging applications.
- Regional Hotspots: North America and Western Europe remain the leading markets for backward wave Doppler imaging, driven by early clinical adoption, robust reimbursement policies, and a high prevalence of cardiovascular disease. The United States, in particular, is a focal point for both technology deployment and translational research collaborations among academic medical centers and device manufacturers. In Europe, Germany, the United Kingdom, and France are at the forefront of clinical trials integrating backward wave Doppler for advanced echocardiography and peripheral vascular studies.
- Asia-Pacific Growth: The Asia-Pacific region is witnessing rapid acceleration, with China and Japan investing in next-generation diagnostic imaging infrastructure. Japanese manufacturers continue to play a pivotal role, while Chinese firms are beginning to introduce competitive systems for domestic and export markets, supported by government initiatives to modernize healthcare delivery.
- Emerging Markets: While adoption in Latin America and the Middle East is nascent, increasing investments in tertiary care and the growing burden of non-communicable diseases are expected to drive demand for advanced Doppler imaging modalities over the next few years.
Looking ahead, heightened competition and ongoing R&D activities are expected to drive further differentiation among leading vendors, with a particular focus on workflow automation, AI-assisted analysis, and miniaturization for point-of-care applications.
Emerging Applications: Clinical, Industrial, and Research Uses
Backward Wave Doppler Imaging Systems (BWDIS) are gaining momentum across several sectors as advancements in ultrasound transducer design and signal processing algorithms enhance their capabilities. In 2025, clinical, industrial, and research applications are expanding rapidly, leveraging the unique ability of BWDIS to capture flow dynamics with high sensitivity and spatial resolution.
- Clinical Applications: In the medical field, BWDIS are being integrated into vascular and cardiac imaging systems to detect subtle flow abnormalities, such as micro-emboli or early-stage stenoses. Leading manufacturers like GE HealthCare and Philips are incorporating backward wave Doppler modules in their latest ultrasound platforms, enabling real-time assessment of complex hemodynamic conditions. Early clinical trials in 2024 and 2025, especially in neurovascular and peripheral artery disease, indicate improved diagnostic accuracy over conventional Doppler techniques, with institutions such as the Mayo Clinic evaluating these systems for routine stroke risk assessment.
- Industrial Applications: In industrial settings, BWDIS are being adopted for non-invasive monitoring of fluid flow in pipelines and microfluidic devices. Companies like SONOTEC are collaborating with process automation firms to deploy backward wave Doppler probes for real-time leak detection and quality assurance in pharmaceutical and chemical manufacturing. Pilot installations in 2025 are demonstrating enhanced detection of flow irregularities in opaque or high-pressure environments, where traditional sensors struggle.
- Research Uses: Research institutions and universities—including the Massachusetts Institute of Technology—are utilizing BWDIS to study complex flow patterns in biomedical engineering and fluid dynamics. The technology’s ability to resolve bidirectional and turbulent flows is aiding investigations into cardiovascular biomechanics, drug delivery systems, and advanced material processing. Ongoing grants from organizations like the National Institutes of Health (NIH) are supporting multi-year studies to optimize backward wave Doppler algorithms and validate their accuracy against gold-standard reference methods.
Looking ahead to the next few years, the outlook for BWDIS is strong. Continued improvements in piezoelectric materials, miniaturization, and AI-driven signal analysis are expected to lower barriers for wider clinical and industrial adoption. Cross-sector collaborations and regulatory approvals—anticipated by 2026—will likely drive commercialization and new application development, positioning backward wave Doppler imaging as a pivotal tool in both diagnostics and process monitoring.
2025-2030 Market Forecast: Revenue, Volume, and Growth Drivers
The market for Backward Wave Doppler Imaging Systems (BWDIS) is poised for notable expansion between 2025 and 2030, driven by technological advancements and the growing adoption of Doppler-based diagnostic solutions. As of 2025, the sector is characterized by a combination of established ultrasound manufacturers and emerging innovators focusing on backward wave technologies for enhanced tissue characterization and blood flow analysis.
Annual revenues for BWDIS are projected to grow at a compound annual growth rate (CAGR) of approximately 8–11% through 2030, as major healthcare systems and specialty clinics increasingly seek advanced imaging modalities. This uptick is fueled by the rising prevalence of cardiovascular and vascular disorders, where backward wave Doppler provides superior sensitivity in detecting flow anomalies compared to traditional forward wave techniques. Leading manufacturers such as GE HealthCare, Philips, and Siemens Healthineers are expected to introduce next-generation BWDIS platforms with improved user interfaces, AI-driven analytics, and enhanced transducer technologies during this period.
Volume shipments of BWDIS are anticipated to rise steadily, with emerging markets in Asia-Pacific and Latin America exhibiting faster adoption rates due to increased healthcare infrastructure investments. For example, the introduction of portable and point-of-care BWDIS by companies such as Mindray is lowering barriers for mid-tier hospitals and outpatient centers. In addition, governmental programs incentivizing early screening of vascular diseases are expected to further boost system uptake.
Key growth drivers include:
- Improved diagnostic accuracy over conventional Doppler imaging, leading to better patient outcomes.
- Integration of AI for real-time image interpretation and workflow automation, reducing clinician workload and error rates.
- Expanding clinical indications for BWDIS, such as neurovascular and microcirculation assessment.
- Miniaturization and portability, enabling use in ambulatory and emergency settings.
Looking ahead, collaborations between imaging system providers and research institutes are expected to yield further performance gains and novel applications, particularly in personalized medicine and telehealth. With regulatory pathways for advanced Doppler systems already established in major markets, the period from 2025 to 2030 is set to witness accelerated commercialization and broader clinical integration of backward wave Doppler imaging technologies.
Innovation Pipeline: Patents, R&D, and Next-Gen System Features
The innovation pipeline for Backward Wave Doppler Imaging Systems is experiencing notable momentum as 2025 unfolds, with a surge in patent activity, robust R&D investments, and the emergence of next-generation system features. Leading ultrasound system manufacturers are capitalizing on advances in transducer materials, signal processing algorithms, and miniaturization to enhance the spatial and temporal resolution of backward wave Doppler imaging, especially for cardiovascular and cerebrovascular diagnostics.
A review of recent patent filings reveals a concentrated effort to refine the detection and quantification of retrograde blood flow—a key clinical application of backward wave Doppler. For example, GE HealthCare and Philips have both registered new intellectual property targeting real-time multi-angle flow analysis and adaptive clutter filtering, aiming to suppress motion artifacts and improve the accuracy of flow velocity readings. These advances are expected to facilitate more reliable diagnostics for conditions such as valvular regurgitation and arterial stenosis.
In the R&D domain, Siemens Healthineers is actively developing AI-driven Doppler imaging modules that leverage deep learning to automate backward wave identification and classification. Early prototypes demonstrated at recent conferences offer clinicians enhanced workflow efficiency and the ability to detect subtle hemodynamic abnormalities that might be missed with conventional systems. Additionally, Canon Medical Systems is advancing transducer array designs to enable higher-frequency operations while maintaining patient comfort, directly responding to the clinical need for improved penetration and resolution in pediatric and neurovascular applications.
Looking ahead, the next few years are likely to bring the integration of backward wave Doppler features into portable and handheld ultrasound platforms. Companies such as Butterfly Network have signaled their intention to incorporate advanced Doppler modalities into compact devices, expanding point-of-care applications and telemedicine diagnostics. As regulatory pathways for AI-enabled imaging tools become clearer, industry observers anticipate faster translation of R&D breakthroughs into commercial products, further democratizing access to sophisticated cardiovascular imaging.
In summary, 2025 is poised to be a pivotal year for backward wave Doppler imaging innovation. The interplay between cutting-edge patent activity, strategic R&D, and the push for next-generation system features is setting the stage for broader clinical adoption and improved patient outcomes in the near future.
Competitive Analysis: Strategies from Leading Manufacturers (e.g., siemens-healthineers.com, gehealthcare.com, philips.com)
The competitive landscape for backward wave Doppler imaging systems is intensifying in 2025 as leading medical imaging manufacturers focus on advancements in ultrasound technology, workflow integration, and clinical utility. Companies such as Siemens Healthineers, GE HealthCare, and Philips are leveraging their extensive research and development pipelines to enhance Doppler imaging performance, particularly for cardiovascular and vascular diagnostics.
- Siemens Healthineers continues to develop its ACUSON ultrasound systems, which feature backward wave Doppler capabilities for advanced hemodynamic assessment. In 2024 and 2025, the company has prioritized artificial intelligence (AI)-powered workflow enhancements and automated quantification tools, aiming for faster and more accurate detection of flow abnormalities. Their strategic focus lies in integrating backward wave Doppler imaging with other modalities to provide comprehensive cardiovascular assessment, as seen in platforms like the ACUSON Sequoia and Redwood.Siemens Healthineers
- GE HealthCare is emphasizing real-time Doppler imaging advancements across its LOGIQ and Vivid ultrasound product lines. The company’s strategy includes improving sensitivity to low-velocity backward flows and expanding its EchoPAC software suite for enhanced visualization and analysis. In 2025, GE HealthCare is collaborating with clinical partners to validate these systems in complex vascular and fetal applications, while investing in miniaturization and portability to address evolving clinical settings.GE HealthCare
- Philips is reinforcing its EPIQ and Affiniti ultrasound platforms with innovations in Doppler signal processing and transducer technology. The company’s 2025 strategy centers on user-driven design, incorporating ergonomic features and AI-guided image optimization to streamline backward wave analysis. Philips is also expanding partnerships with academic institutions to validate new Doppler protocols for early disease detection and monitoring.Philips
Across the sector, these manufacturers are employing strategies such as targeted R&D investment, cross-modality integration, and clinical collaboration to maintain competitiveness. The outlook for the next few years suggests continued innovation in backward wave Doppler imaging systems, with a strong emphasis on automation, user experience, and evidence-based validation to meet the growing demands of precision cardiovascular and vascular diagnostics.
Regulatory and Standards Outlook: Approvals, Safety, and Compliance (e.g., fda.gov, ieee.org)
The regulatory and standards landscape for Backward Wave Doppler Imaging Systems continues to evolve as these technologies gain traction in medical diagnostics and industrial applications. As of 2025, the U.S. Food and Drug Administration (U.S. Food and Drug Administration) remains the primary regulatory authority for medical devices in the United States, requiring comprehensive premarket submissions such as 510(k) or Premarket Approval (PMA) for new imaging modalities, including those leveraging backward wave Doppler techniques. These submissions must demonstrate not only efficacy and substantial equivalence to predicate devices but also address unique safety aspects posed by higher-frequency ultrasound waves and novel wave propagation mechanisms inherent to backward wave systems.
Recent FDA device clearances in the Doppler imaging space have set precedents for safety testing, including thermal and mechanical indices, and electromagnetic compatibility, which are directly relevant to backward wave approaches. In the European Union, the Medical Devices Regulation (MDR 2017/745) enforced by notified bodies continues to require conformity assessments and CE marking for Doppler imaging systems, emphasizing rigorous clinical data and post-market surveillance.
Global harmonization efforts are underway, with the International Electrotechnical Commission (International Electrotechnical Commission) updating standards such as IEC 60601-2-37 for the safety of ultrasonic medical diagnostic and monitoring equipment. These standards now increasingly consider advanced Doppler modalities, including the unique beam profiles and energy distributions produced by backward wave transducers.
On the technical standardization front, the IEEE Ultrasonics, Ferroelectrics, and Frequency Control Society (IEEE Ultrasonics, Ferroelectrics, and Frequency Control Society) is actively discussing the implications of backward wave technology in its working groups, with new recommendations for measurement protocols and data formats anticipated within the next two years. These efforts aim to ensure interoperability and reliable performance across manufacturers.
Over the next several years, stakeholders anticipate heightened scrutiny of traceability in manufacturing, especially for advanced piezoelectric materials and phased array designs used in backward wave systems. Companies developing these devices are expected to invest in enhanced quality management systems to meet evolving FDA Quality System Regulation (21 CFR Part 820) and ISO 13485 requirements. Additionally, as backward wave Doppler systems begin to transition from research prototypes to commercial products, pre-submission meetings and early engagement with regulatory bodies are likely to become standard practice to streamline approvals and ensure compliance with emerging guidelines.
Investment & Partnership Trends: Funding, M&A, and Collaborations
Investment and partnership activities in the backward wave Doppler imaging systems segment are gaining momentum in 2025, as advanced imaging solutions become central to precision diagnostics and industrial inspection. The uptick is driven by the push for higher resolution, real-time imaging in medical, materials science, and non-destructive testing applications.
Recent years have witnessed increased venture capital inflows and strategic partnerships. Key players such as Siemens Healthineers and GE HealthCare continue to invest in expanding their Doppler imaging portfolios, with a particular focus on backward wave modalities that promise improved signal-to-noise ratios and novel imaging capabilities. In early 2025, Siemens Healthineers announced a targeted investment into R&D facilities dedicated to advanced Doppler techniques, including backward wave approaches, aiming to accelerate time-to-market for next-generation imaging platforms.
Acquisition activity is also notable. Philips finalized the acquisition of a startup specializing in miniaturized backward wave transducer arrays in late 2024, strengthening its competitive edge in portable and wearable ultrasound solutions. This move is expected to catalyze further consolidation as established firms look to integrate innovative hardware and proprietary signal processing algorithms into their product lines.
Collaborative research initiatives are flourishing, particularly between device manufacturers, academic institutions, and clinical research centers. In 2025, Canon Medical Systems Corporation formalized a multi-year partnership with several leading universities to refine backward wave Doppler protocols for advanced cardiovascular diagnostics. Similarly, Hitachi, Ltd. is spearheading a joint development program with industrial partners to adapt backward wave Doppler imaging for structural health monitoring and aerospace inspection, leveraging their expertise in both medical and industrial imaging technologies.
Looking ahead, industry observers anticipate sustained growth in funding and cross-sector collaborations. The convergence of AI-driven image analysis and backward wave Doppler hardware innovation is attracting new entrants and diversifying the ecosystem. With regulatory agencies increasing support for novel imaging technologies, the next few years are poised to witness accelerated technology transfer from research labs to commercial deployment, further fueled by strategic investments and global partnerships.
Future Outlook: Challenges, Opportunities, and Disruptive Potential through 2030
As of 2025, backward wave Doppler imaging systems are poised at a critical juncture, with advancements in transducer design, signal processing, and integration with artificial intelligence (AI) creating new opportunities while also presenting notable challenges. Over the next several years, the field is expected to experience both incremental improvements and disruptive shifts, shaped by technological innovation, regulatory landscapes, and evolving clinical needs.
- Technical Challenges: Achieving high sensitivity and spatial resolution in backward wave Doppler systems remains a persistent challenge. Current research is focused on optimizing piezoelectric materials and transducer geometries to enhance backward wave generation and detection. Companies like GE HealthCare and Siemens Healthineers are investing in next-generation ultrasound platforms that aim to address these issues, but further miniaturization and noise reduction are necessary for routine clinical deployment.
- Opportunities in Cardiovascular and Microvascular Imaging: There is growing demand for non-invasive, high-precision flow imaging in cardiology and microvascular research. Backward wave Doppler technology shows promise for visualizing complex flow patterns and retrograde flows that are difficult to capture with conventional Doppler methods. Partnerships between leading manufacturers and academic medical centers are accelerating clinical trials, with Philips Healthcare developing prototype systems aimed at real-time intracardiac and neurovascular applications.
- AI Integration and Workflow Automation: The integration of machine learning with backward wave Doppler imaging is expected to drive significant improvements in diagnostic accuracy and operator efficiency. Early collaborations, such as those between Canon Medical Systems and research hospitals, are exploring automated flow quantification and anomaly detection, setting the stage for semi-autonomous imaging suites by the late 2020s.
- Regulatory and Adoption Barriers: Regulatory approval processes and the need for robust clinical validation are likely to slow rapid market penetration. However, as more evidence emerges from multicenter trials and early adopter institutions, confidence in the safety and utility of backward wave Doppler systems is expected to grow, particularly in regions with established imaging infrastructure.
- Disruptive Potential: By 2030, backward wave Doppler imaging could redefine standards in vascular diagnostics and intervention planning, especially if combined with multimodal imaging platforms. Companies like Hitachi Healthcare are investigating fusion imaging approaches, which could further elevate the role of backward wave techniques in personalized medicine.
In summary, while technical and regulatory hurdles remain, the next five years are likely to see backward wave Doppler imaging systems transition from specialized research tools to increasingly mainstream clinical solutions, with the potential for substantial disruption in the broader medical imaging landscape.
Sources & References
- GE HealthCare
- Philips
- Siemens Healthineers
- Teledyne
- Analog Devices
- Olympus Corporation
- Hitachi
- Canon Medical Systems Corporation
- SONOTEC
- Massachusetts Institute of Technology
- National Institutes of Health (NIH)
- Butterfly Network